產品櫥窗
-
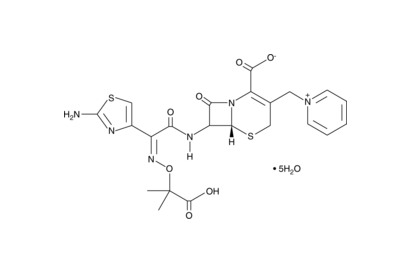
Ceftazidime Pentahydrate(Stock: Inquire)
|
產品型號:6999-78439-06-2 商品規格: |
 |
Broad-spectrum β-lactam antibiotic
C22H22N6O7S2 · 5H2O
MW: 636.65
Ceftazidime pentahydrate is a third generation cephalosporin antibiotic. It has been used to study the penicillin binding proteins, especially PBP3. It has also been used as a selection tool for mutant colonies. Ceftazidime targets gram-negative bacteria, including Pseudomonas aeruginosa and is less effective against gram-positive cocci. It is soluble in acidic and alkali solvents.
Cephalosporins are a type of β-lactam antibiotic consisting of a four-membered β-lactam ring bound to a six-membered dihydrothiazine ring. This two-ring system causes distortion of the β-lactam amide bond, resulting in decreased resonance stabilization and increased reactivity. β-lactams inhibit the formation of peptidoglycan cross-links within bacterial cell walls by targeting penicillin-binding proteins or PBPs. Consequently, the bacterial cell wall becomes weak and cytolysis occurs. Cephalosporins are less susceptible to β-lactamases than the penicillin β-lactam antibiotics.
Stock conc.: 5mg/mL, in 1x PBS. Use immediately.
Gonçalves-Pereira, J., and Póvoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of β-lactams. Critical Care 15(5), R206 (2011).
MW: 636.65
Ceftazidime pentahydrate is a third generation cephalosporin antibiotic. It has been used to study the penicillin binding proteins, especially PBP3. It has also been used as a selection tool for mutant colonies. Ceftazidime targets gram-negative bacteria, including Pseudomonas aeruginosa and is less effective against gram-positive cocci. It is soluble in acidic and alkali solvents.
Cephalosporins are a type of β-lactam antibiotic consisting of a four-membered β-lactam ring bound to a six-membered dihydrothiazine ring. This two-ring system causes distortion of the β-lactam amide bond, resulting in decreased resonance stabilization and increased reactivity. β-lactams inhibit the formation of peptidoglycan cross-links within bacterial cell walls by targeting penicillin-binding proteins or PBPs. Consequently, the bacterial cell wall becomes weak and cytolysis occurs. Cephalosporins are less susceptible to β-lactamases than the penicillin β-lactam antibiotics.
Stock conc.: 5mg/mL, in 1x PBS. Use immediately.
Gonçalves-Pereira, J., and Póvoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of β-lactams. Critical Care 15(5), R206 (2011).
Store at 4oC